Flu Virus binding to Receptor Cells – Credit CDC
# 6865
One of the unresolved mysteries of the 2009 H1N1 pandemic is that while most people saw a relatively mild illness - for a small percentage of the population - the virus proved unusually severe and sometimes deadly.
We saw the burden of the disease shift to younger adults and adolescents, a cohort that normally endures influenza infection pretty well. In Study: Years Of Life Lost Due To 2009 Pandemic, researchers calculated the mean age of death from the pandemic virus to be half that of seasonal flu, or 37.4 years.
And in September of 2011 we saw research indicating the H1N1pdm virus was more likely to exacerbate an S. pneumoniae co-infection (in mice, anyway) than was seasonal H1N1 (see mBio: Lethal Synergism of H1N1 Pandemic Influenza & Bacterial Pneumonia)
During November of 2009, news of a small change in the novel H1N1 virus came by way of the Norwegian Institute of Public Health (see Norway Reports An H1N1 Mutation) who announced the discovery of a mutation that “could possibly make the virus more prone to infect deeper in the airways and thus cause more severe disease."
The announcement of the `Norway’ or D222G (D225G in influenza H3 Numbering) mutation immediately sent researchers around the world on a hunt for similar changes in the virus, and over the following months several variations on a theme were discovered; D222N, D222E, and D222A.
The D222G mutation involves a single amino acid change in the HA1 gene at position 222 from aspartic acid (D) to glycine (G).
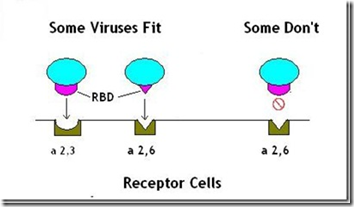
The pdmH1N1 virus carrying this mutation appeared to bind more readily to receptor cells (α2-3) found deep in the lungs, whereas unmutated seasonal flu strains bind preferentially to the (α2-6) receptor cells found in the upper airway.
A virus’s ability to bind to specific cells is controlled by its RBD or Receptor Binding Domain; an area of its genetic code that allows it to attach to, and infect, specific types of host cells.
(A Very Simplified Illustration of RBDs)
Like a key into a padlock, the RBD must `fit’ in order to open the cell to infection.
Similarly, D222A changes the HA1 gene at position 222 to Alanine, while D222E changes the gene to Glutamic acid and D222N changes to Asparagine.
This D222G mutation had actually been detected months earlier, and in several other countries, but Norway was the first country to announce a possible link between that mutation and greater virulence.
In January of 2010, the World Health Organization’s Weekly Epidemiological Record (No. 4, 2010, 85, 21–28) provided a detailed overview of what was then known about this mutation.
While stating that more study was needed, the WHO pointed out the lack of apparent ongoing transmission of this mutation, and stated that:
`Based on currently available virological, epidemiological and clinical information, the D222G substitution does not appear to pose a major public health issue.’
Later in 2010, in Study: Receptor Binding Changes With H1N1 D222G Mutation, we saw more evidence of preferential binding to deep lung cells by viruses with the D222G mutation.
The debate over the significance (and origins) of the D222G mutation have continued since then. You can revisit some of those studies in the following blogs:
Eurosurveillance: Analysis Of Fatal H1N1 Cases In The UK)
All of which serves as prelude to a new study on the D222G mutation – again from Norwegian Institute of Public Health – that appeared yesterday in the journal Eurosurveillance.
Eurosurveillance, Volume 18, Issue 3, 17 January 2013
R Rykkvin, A Kilander, S G Dudman, O Hungnes
Date of submission: 29 December 2012
ABSTRACT (reparagraphed for readability)
The association between a particular mutation in the HA1 subunit of the influenza virus haemagglutinin, D222G, and severe and fatal disease in cases of influenza A(H1N1)pdm09 in Norway during the 2009 pandemic was investigated using pyrosequencing.
The prevalence of the variant among fatal cases was 8/26 and among severe non-fatal cases 5/52. No D222G mutations were found among the 381 mild cases.
This difference could not be attributed to sampling differences, such as body location of sampling, or duration of illness. In cases with mutant virus where clinical specimens from different days of illness were available, transition from wild-type to mutant virus was commonly observed (4/5), indicating that the mutant virus emerged sporadically in individual patients.
In patients with paired samples from both the upper and lower respiratory tract (n=8), the same viral genotypes were detected in both locations. In most of the D222G cases (11/13), the mutant virus was found as a quasispecies.
This a long, and fairly technical report with a lot to digest, and I’m sure many of you will want to read it in its entirety.
But in short the authors present several findings, which they summarize in the discussion portion of the paper:
In the present study, we provide further epidemiological evidence of the association between the D222G mutation in HA1 of influenza A(H1N1)pdm09 virus and severe or fatal clinical course.
Furthermore, we present evidence that the mutated viruses emerge in individual patients after the onset of illness and demonstrate the presence of mutant virus in both the upper and lower respiratory tract. We also address some potential biases that could conceivably confound the analysis.
The Norwegian cases of infection with HA1 222G genotype viruses have occurred sporadically and do not cluster epidemiologically or in phylogenetic analysis.
<SNIP>
The 222G viruses appear to be rare among circulating strains, but are still quite frequent in patients with severe disease, who are not epidemiologically linked. A likely explanation is that the presence of mutant viruses in these particular individuals experiencing severe disease is due to selective upgrowth of mutant genomes during infection.
In other words, this mutation appears to occur spontaneously after a person is infected by the H1N1pdm virus, and then, only rarely. But when that happens, the patient appears more likely to experience a more severe illness.
As previously reported, It does not appear to transmit efficiently in the wild.
The concern here is that viruses can change, and co-mutations could occur that make the D222G mutation more transmissible in the future.
We’ve seen that happen before.
In 2006 we saw a smattering of oseltamivir (Tamiflu ®) resistant seasonal H1N1 cases, almost always attributed to `spontaneous mutations’ within a patient receiving the drug. While of concern to the patient afflicted, it appeared to be poorly transmissible.
In the 2006-2007 flu season, laboratories found no resistant strains in Europe or Japan, and in less than 1% of samples from the United States.
This resistance was caused by a mutation called H275Y, where a single amino acid substitution (histidine (H) to tyrosine (Y)) occurs at the neuraminidase position 275.
(Note: some scientists use 'N2 numbering' (H274Y) and some use 'N1 numbering' (H275Y))
The following year, during the 2007-2008 flu season, oseltamivir resistant viruses suddenly took flight, and by the spring of 2008 roughly 25% of European samples tested showed the H275Y mutation (see Increased Tamiflu Resistance In Seasonal Influenza).
By the end of the year, resistant seasonal H1N1 was pretty much the norm around the world.
Influenza viruses are both unpredictable and constantly changing. So we watch subtle mutations like the D222G carefully, with the knowledge that the limited threat it poses today (due to its poor transmissibility) may not necessarily hold true tomorrow.